Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Hoshi, Harutaka*; Wei, Y.*; Kumagai, Mikio*; Asakura, Toshihide; Morita, Yasuji
Recent Advances in Actinide Science, p.596 - 598, 2006/06
Recently, extraction selectivity for trivalent minor actinides (MA = Am and Cm) over lanthanides (Ln) has been found in some extractants containing soft donor, such as S or N, ligands. Kolarik et al. reported that a new N-donor ligand 2,6-bis(5,6-dialkyl-1,2,4-triazine-3-yl)-pyridine (R-BTP) shows high selectivity for MA (III) over Ln(III) [1]. However, protonation of R-BTP results in its acidic hydrolysis in acidic medium. Stability in acidic solution was improved by substitution of long normal chain or branched chain [2]. In this work, separation of MA(III) and Ln(III) from nitric acid solution was studied by using novel R-BTP impregnated resin. Branched R-BTP resin had high affinity for Am from up to 4 M HNO solution and its distribution coefficient was over 10
solution and its distribution coefficient was over 10 .
.
Nakajima, Kunihisa; Nakazono, Yoshihisa; Arai, Yasuo
Recent Advances in Actinide Science, p.448 - 450, 2006/06
A pyrochemical process for the metallurgical treatment of the spent nuclear fuels contains the Cd-distillation step, where Pu is recovered as a residue. For understanding this Cd-ditillation behavior the samples of PuCd +PuCd
+PuCd and PuCd
and PuCd +PuCd
+PuCd were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd
were prepared and Knudsen-cell mass-spectrometric measurements were carried out to determine the vapour pressures of Cd(g) over these samples. Further,the thermodynamic quantities of PuCd and PuCd
and PuCd were evaluated from these vapour pressures.
were evaluated from these vapour pressures.
Minato, Kazuo; Takano, Masahide; Nishi, Tsuyoshi; Ito, Akinori; Akabori, Mitsuo
Recent Advances in Actinide Science, p.317 - 322, 2006/06
To reduce the radiotoxicity of the high-level waste and to use the repository efficiently, recycling of minor actinides (MA: Np, Am, Cm) as well as plutonium is an option for the future nuclear fuel cycle. For MA-bearing fuel development, new facilities with inert atmosphere were installed and the thermal properties of minor actinide compounds, especially nitrides and oxides, were measured. Minor actinide nitrides were prepared by carbothermic reduction of the oxides. Lattice parameter and its thermal expansion were measured by high-temperature X-ray diffraction, and thermal diffusivity by laser flash method.
Yano, Kimihiko; Shibata, Atsuhiro; Nomura, Kazunori; Koizumi, Tsutomu; Koyama, Tomozo
Recent Advances in Actinide Science, p.644 - 646, 2006/06
U crystallization is effective to minimize solvent extraction process equipment by separating U from dissolver solution of spent fuel. In order to establish crystallization process for FBR MOX fuel, Pu behavior needs to be investigated with mixed U and Pu solution. Pu valence influence on the decontamination factor (DF) of Pu to U in crystal. Pu(IV) was not crystallized with U and DF was about 30. Although Pu(VI) dose not solely crystallized, it was co-crystallized with U and DF of Pu to U in crystal was about 1.3. The effect of Pu(IV) concentration on crystallization yield of U is small. It had a tendency to decrease crystallization yield of U by that Pu(VI) exists, compared with that calculated by solubility of U in U-HNO -H
-H O system.
O system.
 on phase relation
on phase relationOsaka, Masahiko; Miwa, Shuhei; Yoshimochi, Hiroshi; Tanaka, Kenya; Kurosaki, Ken*; Yamanaka, Shinsuke*
Recent Advances in Actinide Science, p.406 - 408, 2006/06
Phase relation of (U,Pu,Am)O solid solution was experimentally investigated by using XRD, ceramograhy and DTA. It is concluded from the present investigation that Am is likely to exist as fixed valences of Am
solid solution was experimentally investigated by using XRD, ceramograhy and DTA. It is concluded from the present investigation that Am is likely to exist as fixed valences of Am and corresponding amount of U
and corresponding amount of U is substituted by the U
is substituted by the U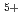 for the preservation of electrical neutrarity.
for the preservation of electrical neutrarity.